It is well known that two of the most iportant properties of zeolites are their microporosity and acidity. The microporososity of these crystalline materials is controlled in the synthesis process according to the pore and channel size distribution of the structure. Channels are window openings formed by 4,6,8,10,12, etc T(Si,Al) atoms. The more T atoms are forming the channel the bigger it is. Channels of a given number of T atoms are slightly different in size according to the structure in which they occur: this is due to the different flexibility of the framework in the different cases. In the figure above (AlPO-5 structure) you can see a view across a 12 MR channel.
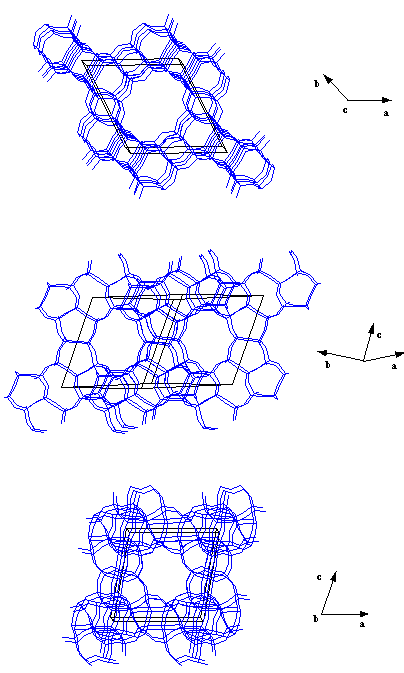
CIT-1 structure.
The MD simulations have carried out with the general purpose DL_POLY code
in its paralell version implemented in a 512 PE CRAY-T3D. The results show
how para-xylene and ortho-xylene diffuse through the 12MR and 10MR channels
systems in [001] and [110] in CIT-1. It is seen how the slightly bigger ortho
isomer can not diffuse through the smaller 10MR channels although some
penetration into the channels is appreciated. The penetration into the 10MR
channels produces a decrease in the diffusivity of ortho-xylene which blocks
itself in these incursions. On the other hand the para isomer diffuses through
both 12MR and 10MR channels in CIT-1. In particular, diffusion through the
10MR channel proceeds quicker due to the impossibility to rotate in these
narrower 'corridors'. Curiously, the 10MR channels are slowing down
the diffusion of ortho-xylene and -at the same time- they are fastening
the diffusion of para-xylene.
CIT-1 presents new and interesting features for the diffusion processes of
C8 aromatics and provides a possible scenario for industrial applications.
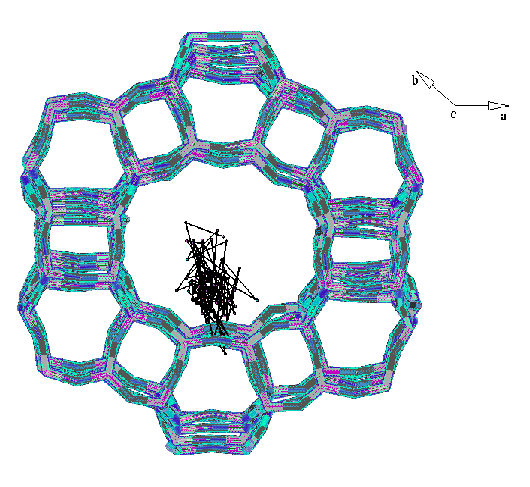
Xylene (centre of mass trajectories) diffusing mainly through the 12MR
channels of the CIT-1 structure.
Activation energies have also been calculated, the results showing that the
para isomer diffuses with very similar activation energies through both
channels, and in the case of the ortho-xylene the activation energy
for the diffusion through 12MR is a bit higher than in the case of 'para'
but still allowing diffusion. In the case of ortho-xylene diffusing
through 10MR channels a very high activation energy, more than 100kcal/mol,
was obtained showing that no diffusion through these channels is possible.
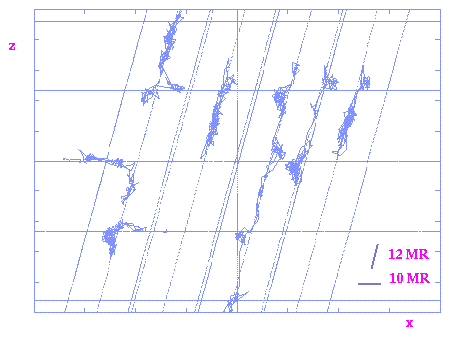
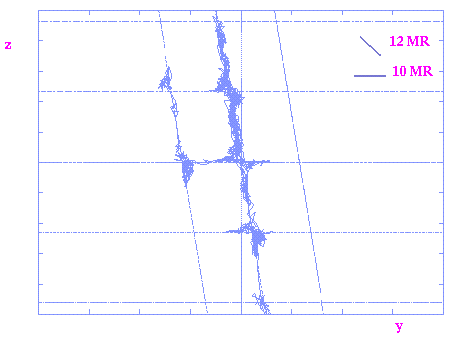
Para-xylene trajectories in a 2-D projection through the CIT-1 structure.
It can be observed in both graphs how there are preferential diffusion in the
bigger 12MR channel but there is also some diffusion in the narrower 10MR
channel. Similar trajectories obtained for the bigger ortho-xylene isomer
show selective diffusion in the 12MR channel only.
This Project was funded by Ministerio de Educacion y Ciencia (Spain)
This postdoctoral project was conducted under Professor
C. R. A. Catlow