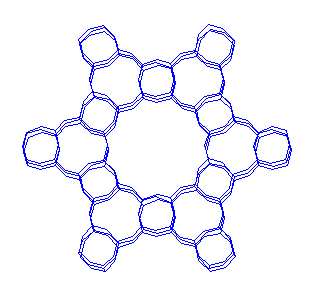
AlPO-5 structure
Initially, the structure of these (crystalline) materials can be considered
as an AlPO (alumino-phosphate) framework...
Some considerations about AlPOs...
AlPO frameworks contain tetrahedrically coordinated Al and P (TO4 units, where T=Al,P), edged linked forming -T1-O-T2- units in which there is a strict structural alternance between Al & P: that means if T1=Al, then T2=P; or to put it another way the first T environments of Al and P are: P(4Al), and Al(4P). This fact is frequently refered to as 'ordered material', because no Al-O-Al, or P-O-P ever appear, as it should happen in a 'random' distribution for Al & P in the network. This has important consequences in the configurational entropy of the material about which I can tell you privately because I don't want to bother you too much.Two more things about AlPOs: they don't have cations to compensate the structure (unlike zeolites) and therefore they don't have either Bronsted acid sites. Consequence of that: they are not very reactive in catalysis. Well, they can have Lewis acidity but you have much better materials in this regard. The second thing is: what about the structure of these materials?. Well, they also microporous like zeolites...
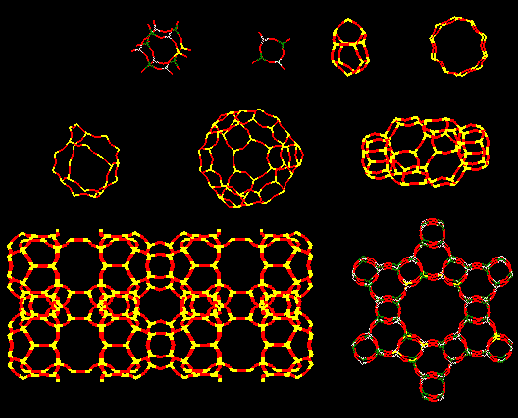
Structure of zeolites. Flexibility of TOT (T=Si,Al) angles allow formation
of different rings with 4, 5, 6, 8, 10, etc members which gives raise to a
virtually infinite different structures with micropores and cavities in the
range 4-20 Angstroms, making zeolites microporous materials.
Some AlPOs have the same structures than some
zeolites (vg. AlPO-5 & SSZ-24; or SAPO-37 & Y; or SAPO-34 & chabazite), but
normally they have unique structures, microporous structures like zeolites but
with different pore systems. Therefore they are potentially attractive as acid
catalysts if we manage to create Bronsted acidity in the structure...
...but how to do that? (That's one of the topics of my research!)
...end of considerations about AlPOs
P-->Si,H and Al,P-->Si,Si substitutions in the framework. Let's see a scheme ...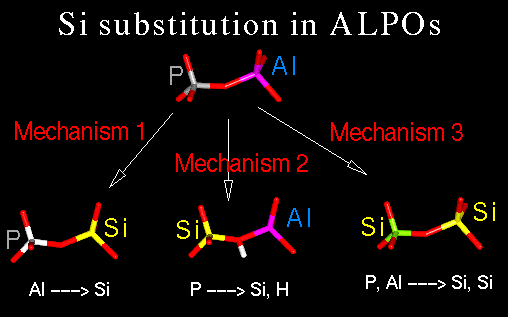
Mechanisms 2 and 3 have been investigated with
computational techniques (Mechanism 1 is not experimentally observed).
The extent of both substitution mechanisms has a relation with the number and
location of the Bronsted acid centers in the material, which are responsible of
the use of the SAPO in acid catalysis.
A better understanding of these mechanisms can provide a good insight in the
selection of appropriate catalysts for industrial processes, according to their
structural and chemical properties.